ExoDx™ Prostate Test
The only exosome-based test that provides unique, actionable intelligence to help you decide if biopsy is necessary; independent of PSA and other standard of care (SOC) features. Connect with a representative in your area for more information today.
% Negative Predictive Value for the ExoDx Prostate Test
% Sensitivity for the ExoDx Prostate Test
% Improved Patient Compliance to Physician Recommendation
% More HGPCa Identified vs. SOC Alone
What is the ExoDx™ Prostate Test?
The ExoDx™ Prostate Test is a simple, non-DRE, urine-based, liquid biopsy test indicated for men 50 years of age and older with a prostate-specific antigen (PSA) 2-10ng/mL, or PSA in the “gray zone”, considering an initial biopsy. The ExoDx Prostate test returns a risk score that determines a patient’s risk of clinically significant prostate cancer (Gleason Score ≥7) on prostate biopsy. A score above the validated cut-point of 15.6 is associated with an increased likelihood of GS≥7 PCa on a biopsy and a score below the cut-point of 15.6 is associated with a decreased likelihood of GS≥7 PCa. This test can help reassure a patient to avoid a prostate biopsy or improve patient compliance with physician recommendation.
What’s Unique about the ExoDX Test
What's unique about The ExoDx test is that it is an exosome-based genomic test that offers an additional data point that is not obtained through the general work up. It offers unique intelligence that is not influenced by DRE, family history, PSA, or other standard of care features.
Clinical Validity
The clinical validity of ExoDx is supported by multiple peer-reviewed publications and presentations, representing approximately 5000 patients from 40 academic and community urology clinics in the US.
The ExoDx Test has been clinically validated to risk stratify clinically significant (Gleason Score ≥7) prostate cancer from low grade prostate cancer (Gleason Score 6) and benign disease. A patient-specific individual risk score is calculated based on a proprietary algorithm that combines the weighted expression of a three-gene signature associated with clinically significant prostate cancer (PCA3, SPDEF, ERG) directly from urinary exosomal RNA.
ExoDx™ Prostate Validation Data
The ExoDx Prostate Test was studied across two clinical trials with 1,022 patients in the United States and has been used in over 30,000 patients since it was first launched.
Study 1
Study 1 (JAMA Oncology, 2016) was conducted as a prospective clinical validation trial between June 2014 to April 2015. The results of the trial were published in JAMA Oncology in 2016.
The study was conducted at 22 clinical sites across the United States and involved some of the country’s leading urologists and prostate cancer researchers from both academic and community-based settings. Study 1 enrolled 1563 participants, of which 519 met the “intended use” population criteria.
ExoDx Prostate Score (EPI) ranges are proportional to increased percent likelihood for HGPCa
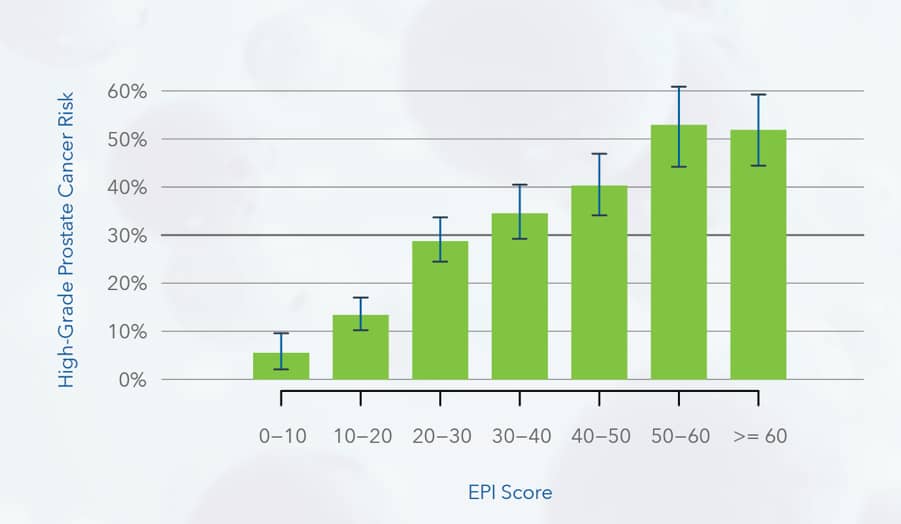
Likelihood of finding HGPCa on biopsy in intended use population
Key Points
- Every patient in this data is in the intended use population: PSA 2-10ng/mL, presenting with decision for initial biopsy, age 50 years and above
- Consider age group relative to EPI risk score and HGPCa risk when interpreting results
The data from the trials demonstrated a statistically significant improvement in the ability to accurately predict high-grade prostate cancer in men presenting for an initial biopsy when added to the standard of care versus standard of care alone (JAMA Oncology, P Carroll et al, July 2016). According to the study findings, the test when added to SOC (defined as, PSA, age, race, and family history) resulted in a statistically significant improvement in the ability to predict high-grade prostate cancer versus SOC alone based on an area under the curve comparison (0.73 versus 0.63; p-value < 0.00004). The authors concluded:
- The ExoDx Test discriminates between high grade (≥ GS7) and low grade (GS6) cancer and benign disease.
- In this study, the urine exosome gene expression assay was associated with improved identification of patients with higher grade PCA among men with elevated PSA levels and could reduce the total number of unnecessary biopsies.
In addition, the test demonstrated a 91.3% negative predictive value (NPV), and a sensitivity of 92% in predicting high-grade prostate cancer biopsy. The study was conducted at 22 clinical sites across the United States and involved some of the country’s leading urologists and prostate cancer researchers from both academic and community-based practices.
Study 2
Study 2 (European Urology 2018) was conducted as a prospective trial in the intended use population, men aged 50 years or more, with a “gray zone” PSA of 2-10ng/mL, considering initial biopsy. The objective of the study was to assess the performance and utility of the ExoDx Prostate Test urine exosome gene expression assay versus standard clinical parameters for discriminating Grade Group (GG) ≥2 PCa from GG1 PCa and benign disease on initial biopsy.
The EPI test performed the same in two prospective validations studies published in top-tier, peer-reviewed journals in over 1000 patients
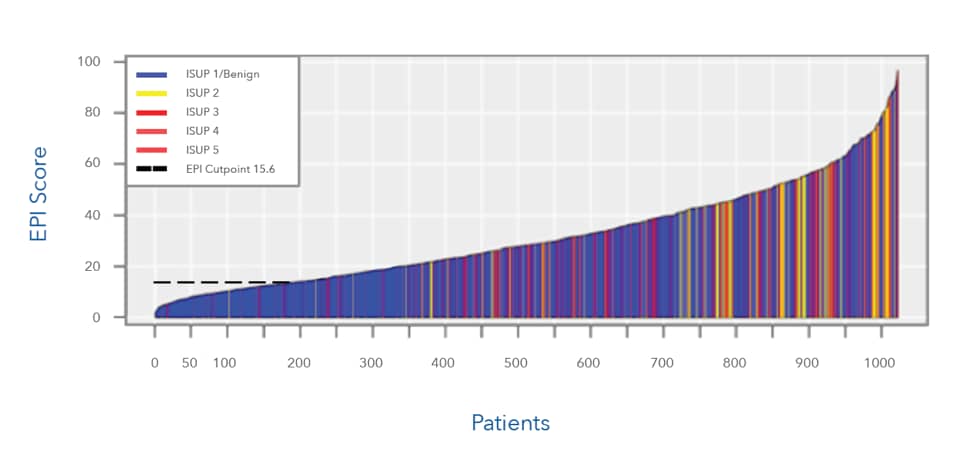
This chart represents >1000 patients who were candidates for initial biopsy. All patients were in intended use population (PSA 2-10ng/mL, presenting with decision for initial biopsy, age 50 years and above
Key Points
- EPI was able to accurately classify patients that were not likely to need a biopsy (Gleason 6/ISUP 1) with a score of 15.6 or less. Note the density of blue below the cut point (indicating ISUP 1/benign)
- EPI was able to accurately classify patients that were more likley to need a biopsy (Gleason 7/GG2) with a score of 15.6 or higher. Note the high density of yellow and red color above the cutpoint, indicating Gleason 7 and above (thus indicating higher grade group and need for biopsy)
- ISUP 1/benign: Gleason 6
- ISUP 2: Gleason 7 (3+4)
- ISUP 3: Gleason 7 (4+3)
The authors conclude:
- ExoDx is a non-invasive, easy-to-use, gene expression urine assay, which has now been successfully validated in over 1000 patients across two prospective validation trials to stratify risk of ≥GG2 from GG1 cancer and benign disease.
- The test improves identification of patients with higher grade disease and would reduce the total number of unnecessary biopsies.
Key Milestones
Based on compelling results from these two well-designed prospective clinical validation studies and an ongoing innovative utility and cost-effectiveness trials conducted in collaboration with CareFirst BlueCross BlueShield, both private and public payors have made positive coverage decisions for The ExoDx Test.
- The National Comprehensive Cancer Network (NCCN) include ExoDx in the May 2019 Prostate Cancer Screening Guidelines (NCCN V2.2019) for early detection of prostate cancer in men for both initial and prostate biopsy (www.nccn.org)
- National Government Services (NGS) has retired Local Coverage Determination (LCD) L37733 effective March 1, 2024, which was specific to Exosome Diagnostics’, a Bio-Techne brand, ExoDx Prostate Test (EPI). Reimbursement guidelines for EPI are now contained in the Centers for Medicare and Medicaid Services (CMS) Billing and Coding Article A56609 - Billing and Coding: Biomarker Testing for Prostate Cancer Diagnosis which was revised effective March 1, 2024.
- According to Centers for Medicare and Medicaid Services (CMS) Billing and Coding Article A56609, the CPT codes in Group 1 are considered medically necessary when ordered by a physician or other qualified health care professional (i.e., NP, CNS, PA). 4K Score should be billed with 81539, PCA3 should be billed using code 81313, ConfirmDx should be billed using code 81551, %fPSA should be billed using codes 84153 and 84154, PHI should be billed using codes 84153, 84154 and 86316, SelectMDX should be billed using code 81479 and the ExoDx Prostate Test should be billed using code 0005U. If a Z-Code is required, Z02Y6 is assigned to the ExoDx Prostate Test.
Connect with Us
Request a MeetingRequest ExoDx Prostate kits for your practice today!
Join our Mailing List!
Stay InformedReceive the latest updates on our scientific advances and get invited to educational events.

